FDA Tobacco Substantial Equivalence – Could your favorite brand be banned?
In a conference call with the tobacco industry (and me) earlier today, FDA announced they would begin to enforce the Substantial Equivalence provisions of the Family Smoking Prevention and Tobacco Control Act (TCA). Essentially this means that any tobacco product including cigarettes, snus, snuff, ryo tobacco, and any other product which was not commercially available in the US as of Feb 15 2007 and/or has been “changed” by the manufacturer can be pulled off the market by FDA as of March 23, 2010. In order to prevent this, manufacturers selling tobacco products in American stores must comply with the TCA and FDA rules by completing and submitting one of the following prior to March 23, 2010:
- Submit to FDA a pre-market application under section 910(c) and obtain an order authorizing marketing of the new tobacco product (section 910(c)).
- Submit to FDA a Substantial Equivalence Report and obtain an order finding the product to be substantially equivalent to a predicate tobacco product (section 910(a)(2)).
- Submit to FDA a request for an exemption from the Substantial Equivalence requirements and obtain an exemption (section 905(j)(3))
FDA stated in its Guidance Document that “All new [post-February 2007] tobacco products regulated by the Food and Drug Administration (FDA) are subject to these provisions. Currently, FDA’s Center for Tobacco Products (CTP) regulates cigarettes, roll-your-own, and smokeless tobacco products. However, the law permits FDA to deem other tobacco products to be subject to the Tobacco Control Act through rulemaking.”
How the FDA Tobacco Substantial Equivalence Rules could Ban your favorite snus or tobacco product 11 Weeks from now
Switching back to plain English, FDA considers anything with tobacco in it (except cigars, which Congress exempted from the TCA…for now) is potentially subject to Substantial Equivalence. This includes every product which is/will be considered for a Modified or Reduced Risk designation from FDA and TPSAC. That includes real snus.
While PACT Act and Kennedy/Waxman were the buzzwords and demons of 2010, the watch-words tobacco consumers need to fear and pay very close attention to in 2011 are Substantial Equivalence and Predicate Product. How FDA chooses to define these words will determine if your tobacco product/brand of choice will still be available in US stores or will have been pulled off the shelves by FDA this spring.
In a rational world, if a manufacturer marketed cigarettes, snus, or any other tobacco product prior to Feb. 2007, they should be able to accurately state that these are Predicate Products to their new tobacco products of the same type.
Simply put; since Marlboro cigarettes and General Snus were commercially marketed in the US prior to 2007, current Marlboro cigarettes and General Snus would be grandfathered as well. All Altria and Swedish Match should have to do is file option #2 above by submitting a Substantial Equivalence Report and FDA would quickly issue a rubber-stamped finding approving all current products under these respective brand names for sale in the US.
In the case of Swedish and Scandinavian snus US (barely) over-the-counter brands such Thunder, Offroad, Gotlandssnus, Catch, Jakobsson’s, and soon Skruf, their manufacturers should be able to establish that their products are substantially equivalent to Swedish Match’s General Snus and thus be permitted.
Since all the current Swedish Match brands of snus are made to the same GothiaTek quality standard that General Snus is, the Ettan, Grov, Goteborg’s, 0102, Röda Lacket, Tre Ankare, Kronan, Probe, Click, and Nick & Johnny Swedish Match brands could technically be introduced and sold over-the-counter as soon as the applications hit FDA’s desk.
Reynolds should have it even easier with Camel SNUS. While “modern” Camel SNUS was not commercially launched in the US until July 2007, the original round can Camel Snus made for Reynolds by BAT was test-marketed in Austin and Portland back in April 2006. Logic would seem to dictate that today’s Camel SNUS products are substantial equivalents of Camel Snus 2006.
After all, a cigarette is a cigarette and snus is snus in the broad sense, right? Unfortunately, not to FDA.
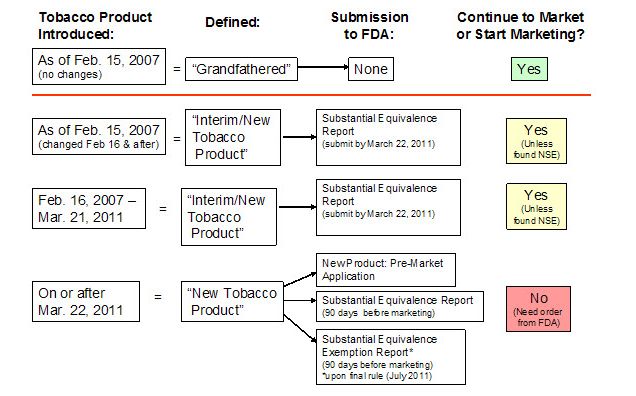 Under then-Kennedy/Waxman; now the Family Smoking Prevention and Tobacco Control Act of 2010 (TCA), all tobacco manufacturers selling over-the-counter in the United States first had to conform to the new labelling requirements and register their products with FDA by June 22nd of 2010.
Under then-Kennedy/Waxman; now the Family Smoking Prevention and Tobacco Control Act of 2010 (TCA), all tobacco manufacturers selling over-the-counter in the United States first had to conform to the new labelling requirements and register their products with FDA by June 22nd of 2010.
It was a little uncomfortable for the manufacturers since registration included listing all the ingredients used in their products as well. Since they did not have to supply the quantity of each ingredient and all this information was/is confidential between each manufacturer and FDA, most registered. After all, food products in the US contain ingredients listings. Just knowing what is in Coca Cola doesn’t mean you can make it yourself in your bathtub.
The Substantial Equivalence Rule is a lot more granular. It does require amounts of all ingredients and constituent parts used in a tobacco product. A cigarette or snus made 10 years ago may not have exactly the same formulation today. In fact, aside from menthol, every flavored snus available today could be considered a different product than their predicate products. Can we even assume that portion, white portion, long portion and mini portion snuses are all predicated by General Loose Snus or round can Camel SNUS?
Is all the above just my normal paranoia setting in? Not according to Kevin Altman of the Council of Independent Tobacco Manufacturers (CITMA). Both of us were on today’s FDA call and we spoken extensively afterwards.
Mr. Altman believes the Substantial Equivalence Rule “will have a devastating effect on many tobacco companies”. While he believes the small to medium sized manufacturers are most at risk, Reynolds, Altria and Lorillard should be worried too.
When FDA was busy writing the rules for tobacco, the Medical Devices industry (FDA regulated) already had in place a Substantial Equivalence model. It differs from the Tobacco Substantial Equivalence Rule in two key areas. First, while tobacco contents and constituent parts are confidential, those used in medical devices are public record. This allows medical device manufacturers to pick the their predicate product much more accurately.
More interesting, there is an allowance for “hybrids”; referencing this part of a product from one manufacturer and another part from another manufacturer to create a predicate product.
Sticking to Swedish Snus since that does affect me personally, lets apply the Medical Devices Industry rules to tobacco. Post-February 2007 snus products from different manufacturers could be submitted using General snus as a Predicate Product so long as the ingredients listed for General snus were very close to their own. [I know Swedish Match and any other snus or tobacco product manufacturer would never agree with this information being publicly available to their competitors, but humor me for the sake of this argument].
To continue, if there were some additional common ingredients/constituents in the pre-2007 Camel SNUS made by BAT, their application could be further justified by stating theirs was a hybrid snus based on both General and Camel. Either way, since all tobacco product ingredients would be public knowledge, they could quickly fine-tune their application into a version most probable to be quickly and rightly approved by FDA.
If justifying flavored snus became necessary this could be done by adding other qualified products to their hybrid model application. Flavor would be defined as flavor in general; not a specific type of flavor. This is certainly a much easier and compliance-friendly model.
Altman suspects that Medical Devices Predicate Product rules are considered by some in government to be a little too manufacturer friendly. If true, that could be an additional factor in why FDA is making the Tobacco rules so much more onerous.
Accepting that, I still believe the strong anti-cigarette/anti-all-tobacco culture at FDA resulted in the following response to a question during the call. FDA’s Kathleen Quinn stated that Substantial Equivalence Rules for Medical Devices rules were not used for Tobacco…..her answer basically boiled down to “because FDA doesn’t want to”.
Added to the mix is FDA’s slight clarification concerning grandfathering of pre-Feb. 2007 tobacco products.
As usual with anything tobacco related, FDA has created a short compliance window with incomplete guidance on a critical issue. Jim Dillard of Altria asked if FDA could provide much more guidance concerning the Exemption Rule prior to Mar 23rd or allow manufacturers to supplement their applications as more guidance became available?
FDA Tobacco Czar Dr. Lawrence Deyton did respond to Altria’s question. He stated that since the time-frame was so short and the current FDA Guidance left a lot of questions unanswered, any manufacturer who submitted a “credible and sincere” application on or before March 22nd would be granted some leeway in amending/supplementing their application if need be.
Deyton did not in any way suggest that amending or adding to their applications would ensure they would be approved by FDA, however. He also neglected to define what “credible and sincere” meant in turns of content, examples, data or who in FDA would be deciding that.
Regarding Substantial Equivalence, there is also a “case by case” reference in the FDA Guidance materials. Does this mean each FDA person reviewing the three types of applications will be applying their own personal standards as to what constitutes “credible and sincere”?
Not explained at all by FDA was why this process was being so rushed out in the first place….and with FDA denying a Public Comment period. Yes, the TCA does have Congressional-mandated benchmark dates for certain events to occur.
But if FDA and the Tobacco Industry agree that the public good would be better served by Congress extending certain implementation benchmarks, extensions could be tacked on as riders to any piece of legislation coming up for a vote at the time. Quality of tobacco regulations doesn’t seem to be driving FDA as much as the sheer quantity they can rush out.
Key provisions of the TCA and FDA tobacco rules are already making their way through the US Court system. What a waste of time, taxpayer money, and resources. Do the job right the first time and many of the legal challenges would never have occurred in the first place. Congress and the President actually reading the entire TCA cover to cover before rushing it into law would have helped too.
If the strong anti-tobacco sediments of FDA’s Tobacco Products Scientific Advisory Committee (TPSAC) are representative of the other FDA tobacco bureaucrats; which is pretty much universally accepted as being the case, then how could almost anything submitted on tobacco company letterhead be considered to be “sincere”.
Grandfathering of pre-2007 tobacco products is not even a slam-dunk under the Substantial Equivalence Rule effective March 23rd. The rule language states it applies to all tobacco products (except cigars) which were commercially marketed in the US after Feb 15 2007 OR were “changed” in any substantive way since that date. With the new reporting requirements, FDA will now have much more detailed product information consisting of not only the product ingredients/constituents but the amounts of each used.
What does “changed” mean to pre-2007 tobacco products? Effectively, whatever FDA wants it to mean: there are no hard standards. It could mean General Mint White Portion and General Mini Mint could be pulled from US stores, at least for a time. The same holds true for Camel Crush cigarettes and all other Swedish/Scandinavian snus products currently in US stores. The list goes on. I hope these worst case scenarios are just that and March 23rd will come and go leaving the nicotine addicted and tobacco lovers alone. I doubt it will, though.
In either event, Kevin Altman of CITMA states very strongly that the biggest challenge for all tobacco product manufacturers; especially new (to the US market), small and medium sized manufacturers will be to “Find the Predicate Product FDA will accept”.
New Tobacco Products in the US market have another challenge.
Dr. David Ashley of FDA added another set of hurdles when it comes to adding new tobacco products or making substantial changes to existing brands. Manufacturers will have to submit a risk/benefit statement demonstrating how these products will be no more harmful to tobacco users and non-users than existing products.
This at first blush sounds fair enough. Dr. Deyton’s statement on the subject is a somewhat reasonable though skewed: “No known existing tobacco product is safe, and a market order issued by the FDA for these products should never be interpreted as such” he stated. “One of the FDA’s missions required by this new law is to ensure new products do not pose an increased threat to the American public. These products will not be safer, but we are required by this law to not allow even more dangerous products to cause further harm to those Americans who use tobacco products.”
I wish Dr. Deyton would apply the same argument to Big Pharmaceutical NRT products; especially Chantix. There is now enough documented evidence to prove the Chantix pill for quitting cigarettes causes depression, suicidal thoughts and documented suicides in people who had no such mental health history before taking Chantix.
FDA has not pulled Chantix from the market; they have reclassified Chantix as a Black Box product meaning it can be very dangerous and physicians should use it as a last resort or very carefully monitor their patients on Chantix.
I really wish FDA would pull the “happy-ending success stories from “real” people” commercials which pop up on television regularly. In my opinion, Chantix does represent a substantially more harmful product than tobacco to non-users of Chantix now. The commercials are misleading and directly target non-users of Chantix.
Most importantly, Chantix commercials as seen on my 82″ TV make me want to throw my snus can at the screen. That would be a very bad thing to have happen and very expensive to correct. If I were actually taking Chantix at the time, I might even jump off a roof in dispair.
I mention Chantix only to demonstrate that while laws may be well intentioned, it’s the interpretation and rules generated by the enforcing agency; in this case FDA, which makes all the difference.
Few in the tobacco industry are opposed to government oversight of the tobacco industry. Sweden has regulated snus as a food product for over 40 years. The results have been positive for both Swedish snus consumers and manufacturers.
This is not the tact FDA is taking with tobacco, however. Their culture is decidedly anti-tobacco and the devil is in the details.
Respected Anti-Cigarette/Pro-NRT products activist Bill Godshall would define a new tobacco product’s dangers to users and especially non-users very differently than Anti-All-Tobacco Zealot Matt Myers of the extremist group Committee for Tobacco Free Kids.
If a snus manufacturer attempts to launch or has a post-2007 Strong or Extra Strong nicotine snus for over-the-counter sale in the United States, will FDA suddenly claim that higher nicotine makes the snus more dangerous than existing grandfathered products? Will high nicotine Swedish snus only be available over the Internet from eStores with no US physical presence and Duty-Free shops again?
While the Tobacco Control Act restricts FDA from banning nicotine in tobacco products, anti-tobacco zealots are already talking about mandating the reduction of nicotine to such a low level that the effect would be the same as the products having no nicotine at all. Nothing is off the table for the anti-tobacco zealots and tobacco Substantial Equivalence rules just provide them another opportunity to pursue their scorched earth agenda against all American tobacco users and nicotine addicts.
The War on Tobacco Continues
There are other dangers to discuss in future stories but if you only take two things away from this article, let them be these:
1. Pay very close attention the words Substantial Equivalence and the date March 23rd, 2011.
2. Never forget that nicotine addicts and tobacco lovers are hated by anti-tobacco extremists. They don’t care about reduced harm, reduced risk, freedom of choice or personal freedom in America. They are driven and blinded by their emotions and personal agendas.
Even if the tobacco products in question have only 1% of the risks associated with cigarettes, the zealots consider them evil. They don’t care if in addition, snus is completely discreet, involves no spitting, environmentally friendly, and is completely harmless in every way to non-snus users…….especially “the children”.
They are patient and have been fighting this war against tobacco for over 40 years now. They will never stop and never surrender to common sense. Remain vigilant and engaged; for your sake, America’s sake, and the sake of your children. Welcome to Amerika 2011. It’s still not a bright day for America’s founding principles.
Guarding our Snus,
LARRY WATERS
FORMER Smoker; CURRENT Swedish Snus User
Reporting From SnusCENTRAL.org
About author
You might also like
Swedish Match Snus Store in Gothenburg Grand Opening!
Today marks the Grand Opening of the latest Swedish Match snus store in Gothenburg. It joins the original in Stockholm and store in Strömstad as showcases of the Swedish Match
EU Health Commissioner John Dalli ousted in EU Snus Corruption Scandal
EU Health Commissioner and snus hater John Dalli has resigned in a snus corruption scandal. Long a roadblock in Sweden’s attempts to have the EU Snus Ban dropped from the
Has The Northerner bit off more than it can snus?
The price of Swedish snus at the major eStores has always varied to one degree or another but never to the extent seen today. Factors such as the PACT Act,


